Virucidal Efficacy
Virucidal activity of disinfectants and sanitizers products prevent diseases caused by enveloped and non-enveloped viruses from spreading.
We validate the virucidal efficacy of critical and non-critical articles; sanitizing surfaces and textiles; and disinfectant equipment.
What is cytophatic effect?
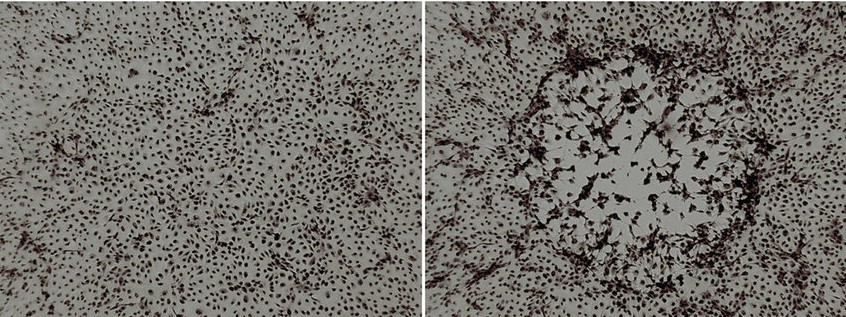
Cytopathic effect (CPE), structural changes in a host cell resulting from viral infection. CPE occurs when the infecting virus causes lysis (dissolution) of the host cell or when the cell dies without lysis because of its inability to reproduce.
How to measure virus viability?
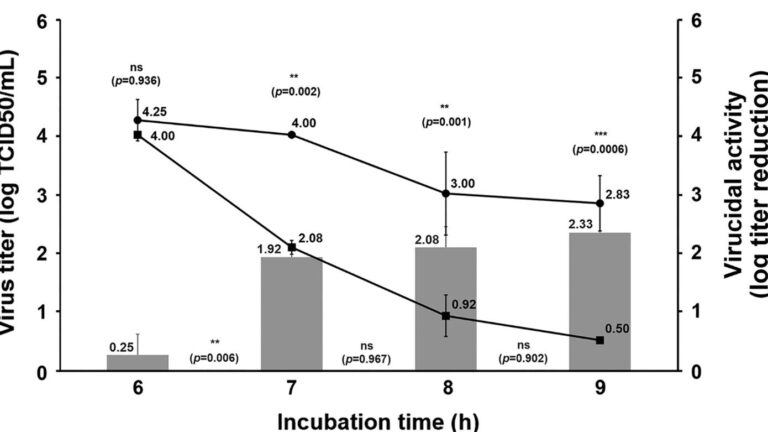
TCID50 (Median Tissue Culture Infectious Dose) assay is one method used to verify the viral titer of a testing virus. Host tissue cells are cultured on a well plate titer, and then varying dilutions of the testing viral fluid are added to the wells.
Our references:
EN14476:2019 – Quantitative suspension test for evaluation of virucidal activity in the medical area for hand sanitizers;
ISO 21702:2019 Measurement of antiviral activity on plastics and other non-porous surfaces;
ISO 18184 – Determination of Antiviral Activity of Textile Products
Our methods
We are a leading laboratory in antiviral efficacy studies, using methods such as:
- Viral titration and cytopathic effect;
- Genetic material damage through RT-qPCR;
- Viral replication inhibition (CALU-3 cells).
Our virus bank:
- COVID-19 virus: SARS-CoV-2 (B.1.1.17);
- Omicron (B.1.1.529);
- Delta (B.1.617.2);
- Vaccinia virus ATCC VR-157;
- Murine Norovirus;
- Adenovirus;
- Enterovirus;
- Filoviridae;
- Flavivirus;
- Herpesviridae;
- Hepatitis A virus (HAV); Hepatitis B virus (HBV).
