Biological Products
Biological products are defined by the Food and Drug Administration (FDA) as products used to diagnose, prevent, treat, and cure diseases and medical conditions.
Biological products are generally large, complex molecules, and compose a wide range category of products, including vaccines, blood and blood components, allergenics, somatic cells, gene therapy, tissues, and recombinant therapeutic proteins.
All of our in vitro pre-clinical tests for biological products are based on FDA; European Medicines Agency (EMA); and European Standard (EN) resolutions:
Mycoplasma detection
Detection of the presence of gDNA with specific primers for 16S-23S intergenic regions of Mycoplasma species with the highest incidence of cell culture contamination (M. arginini, M. fermentans, M. hominis, M. hyorhinis, M. orale, M. salivarium , M. pirum, A. laidlawii) using the qPCR technique.
Reference: FDA Points to Consider in the Characterization of Cell Lines Used to Produce Biologicals.
Sterility test
Detection of anaerobic bacteria, aerobic bacteria, yeasts and fungi through the inoculum of samples in Thioglycolate and Soy Casein Broth.
Reference: Brazilian Pharmacopoeia, 6th edition sterility test (5.5.3.2.1)
Endotoxin analysis
Semiquantitative detection of endotoxins present in cell culture supernatant using the Gel Clot Limulus amebocyte lysate (LAL) method.
Reference: FDA Points to Consider in the Characterization of Cell Lines Used to Produce Biologicals.
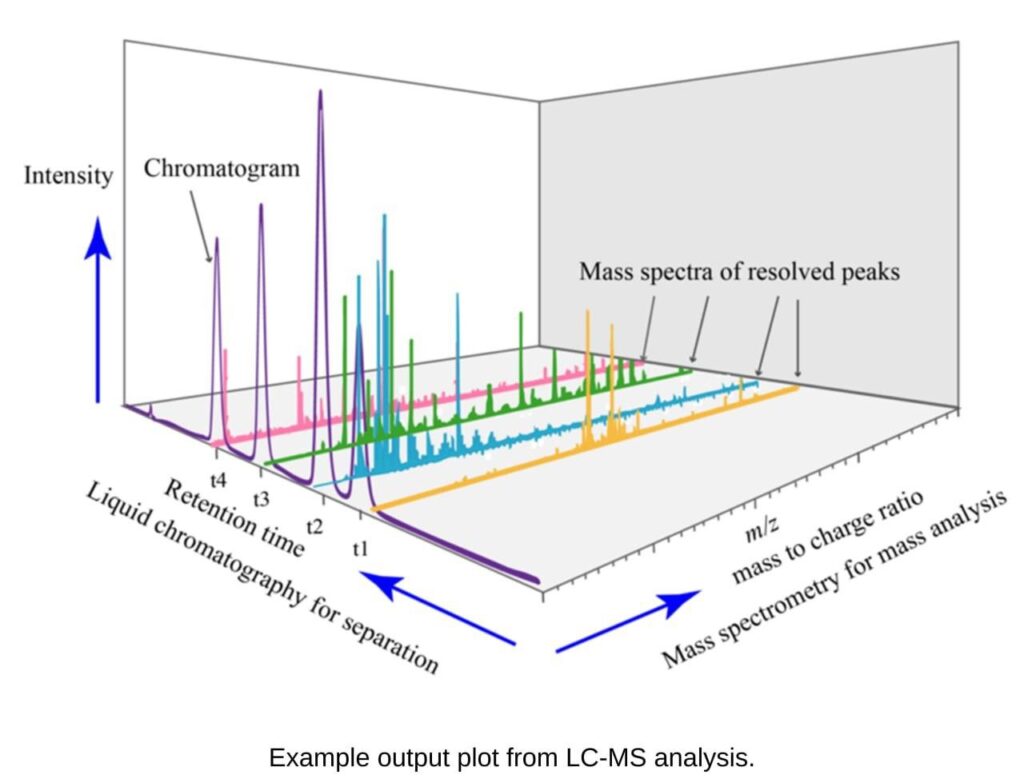
Quantification of impurities and metabolites
Detection and quantification of lactate, glutamine/glutamate, ammonia and glucose in cell culture supernatant samples, through Liquid chromatography–mass spectrometry (LC–MS).
Reference: EMA – ICH Topic Q3: Impurities. (Including guidelines on “Impurities in New Drug Substances,” “Impurities in New Drug Products,” and “Impurities: Residual Solvents”).