Viral infections represent a significant global health challenge, affecting millions of people every year. The rapid spread of viral illnesses such as influenza, HIV, hepatitis C, and the Ebola virus highlights the need for effective antiviral treatments. In this context, the research and development of molecules with antiviral activity has been an area of intense pharmaceutical scientific investigation. This article will discuss recent advances in the discovery and development of antiviral drugs, highlighting their effectiveness in fighting viral infections.
Virus Life Cicle
To understand how to inhibit viral action, you must first understand it. Before addressing the mechanisms and development of antiviral molecules, let’s recall the main aspects of the viral life cycle.
Viruses are intracellular parasites that in their life cycle must penetrate host target cells and take control of their cellular machinery. The viral lifecycle is made up of three distinct phases, including entry, genome replication, and exit, as shown in the following figure.
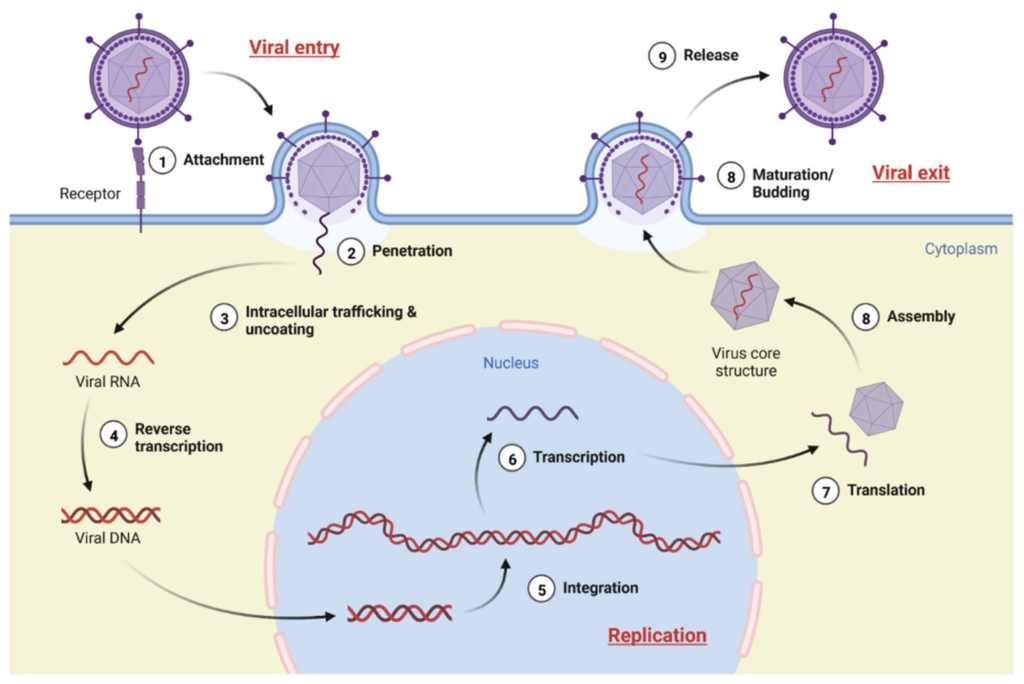
https://doi.org/10.3390/molecules27134305
Each of these phases can be inhibited by the action of antiviral molecules and that is what we will discuss below.
Antiviral mechanisms:
To understand the effectiveness of antiviral molecules, it is crucial to know in depth the mechanisms by which they act.
Antiviral molecules can have different targets within the viral life cycle, including inhibition of virus entry into the host cell, blocking viral replication, inhibiting viral assembly, or interrupting the release of viral particles. In addition, some antiviral molecules stimulate the host’s immune system, strengthening its natural antiviral response.
- Inhibition of viral entry:
Many viruses rely on specific interactions between viral proteins and receptors on host cells to enter and infect the organism. Antiviral molecules can act by inhibiting these interactions. One example is enfuvirtide, a drug used in the treatment of HIV, which prevents the virus from merging with the cell membrane, blocking its entry into CD4+ cells.
- Inhibition of viral replication:
Viral replication is an essential process for virus dissemination in the host organism. Antiviral molecules can target key steps in this process. Polymerase inhibitors, for example, are used in the treatment of hepatitis C infections and act by inhibiting the viral polymerase enzyme, which is essential for the synthesis of the virus’s genetic material. This inhibition prevents viral replication and reduces the viral load in the body.
- Inhibition of viral assembly:
After viral replication, new viral particles are assembled to form mature virions that are released from host cells. Antiviral molecules can interfere with this process. For example, protease inhibitors are widely used in the treatment of HIV. They block the activity of the viral protease enzyme, preventing the processing of viral proteins and the formation of infectious viral particles.
- Stimulation of the immune response:
Another important antiviral mechanism is the stimulation of the host’s immune response. Some antiviral molecules can increase the activity of the immune system, strengthening its natural antiviral response. This may involve activating immune cells such as T lymphocytes and natural killer (NK) cells, which play a crucial role in eliminating virus-infected cells.
It is important to highlight that these mechanisms may vary according to the type of virus and the class of antiviral molecules used.
Development of antiviral drugs:
The development of antiviral molecules involves a series of steps, from identifying promising viral targets to preclinical and clinical evaluation of efficacy and safety. Advances in high-throughput screening techniques and computational modeling have accelerated the discovery of molecules with antiviral activity. In addition, an in-depth understanding of viral biology and virus-host interaction has been instrumental in identifying new therapeutic targets.
Role of preclinical trials in antiviral drug development:
Preclinical studies play a key role in the development of drugs with antiviral molecules, providing essential information about the efficacy and safety of these substances before they are tested in humans. These studies are performed in laboratories and animal models to assess the therapeutic potential of antiviral molecules and lay the groundwork for subsequent clinical trials.
Among the main contributions of preclinical studies in the development of drugs with antiviral molecules are:
- Assessment of antiviral activity:
Preclinical studies allow the evaluation of the antiviral activity of molecules against different types of viruses. The researchers perform in vitro tests to determine whether the molecules can inhibit viral replication and reduce viral load. This provides early evidence of the molecules’ potential effectiveness in fighting viral infections. Some of the in vitro techniques that can be used for this evaluation are:
- Cytotoxicity Assays: These assays are used to assess whether antiviral molecules have direct toxicity to host cells. Techniques include vital dye staining assays such as the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay and the trypan blue exclusion assay.
- Immunofluorescence Assays: These assays are used to visualize the presence of viral antigens on infected cells and to evaluate the effectiveness of antiviral molecules in inhibiting viral replication. Specific antibodies are used to identify the presence of viral proteins.
- Quantification of viral load by real-time PCR: This technique allows precise quantification of viral RNA or DNA present in samples. It is used to evaluate the effectiveness of antiviral molecules in reducing the viral load in infected cells.
- Plate Culture Assays: In these assays, cells infected by the target virus are cultured in culture plates and the formation of viral plaques is observed. The presence of antiviral molecules is assessed by inhibiting plaque formation or by reducing the number of plaques formed.
- Toxicology and safety:
Preclinical studies are also crucial for evaluating the toxicity and safety of antiviral molecules, identifying possible adverse effects of the substances, and providing fundamental information for establishing the safe and effective dose of the drug and identifying any potential health risks. Some of the in vitro techniques that can be used in this evaluation are:
- Cellular Toxicity Assays: These assays are performed to assess the effects of antiviral molecules on healthy cells. Some examples of techniques used are the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay, the trypan blue exclusion assay, and the lactate dehydrogenase release assay ( LDH).
- Genotoxicity studies: In vitro genotoxicity tests assess whether antiviral molecules can damage the genetic material of cells. Some commonly used techniques are the micronucleus test, the bacterial mutation test (such as the Ames test), and the DNA repair assay test.
- Pharmacokinetics and bioavailability:
Preclinical studies also investigate the pharmacokinetics of antiviral molecules, that is, how the drug is absorbed, distributed, metabolized, and excreted by the body. This information is important in determining the proper dosage and frequency of drug administration. In addition, pre-clinical studies help to identify the bioavailability of molecules, that is, the amount of medication that effectively reaches the site of action in the body.
Among the in vitro techniques that can be used to evaluate the pharmacokinetics and bioavailability of drugs are:
- Cellular uptake studies: These studies use cultured cells to assess the uptake of antiviral molecules. Cell permeability assays and active or passive transport of substances are carried out.
- In vitro liver metabolism: Isolated liver cells or specific cell lines are used to assess liver metabolism of antiviral molecules. Assays such as the Liver Microsome Assay and the Hepatocyte Suspension Assay are commonly used.
- Plasma protein binding: This technique assesses the affinity of antiviral molecules for plasma proteins, such as albumin. In vitro protein binding assays are performed to determine the percentage of molecules that bind to proteins.
- Selection of candidates for clinical trials:
Based on the results of preclinical studies, researchers can select the most promising antiviral molecules to move forward to human clinical trials. Preclinical studies provide crucial data that help inform the choice of the most suitable candidates for the next phase of development, taking into account both the efficacy and safety of the drugs.
Some of the in vitro techniques that contribute to this selection are:
- In Vitro Antiviral Efficacy Assays: Cultures of cells infected with the target virus are used to assess the effectiveness of antiviral molecules in inhibiting viral replication. Viral load quantification assays, cytotoxicity assays, and viral gene expression inhibition assays are performed.
- Non-infected cell toxicity assays: These assays evaluate the effects of antiviral molecules on healthy cells not infected by the target virus. Cell viability assays, oxidative stress assays, and apoptosis assays are used to assess the toxicity of substances.
It is important to emphasize that in vitro techniques may vary depending on the target virus, the type of cell used, and the specific objectives of the preclinical study.
Preclinical studies are essential for the development of drugs with antiviral molecules, providing critical information about the antiviral efficacy, toxicity, pharmacokinetics, and safety of these substances.
These studies are fundamental to support decision-making and the selection of the most promising candidates for clinical trials, taking us closer to the development of effective and safe antiviral treatments.
Examples of effective antiviral molecules:
In recent years, several antiviral molecules have demonstrated efficacy in the treatment of specific viral infections. A notable example is the use of protease inhibitors for the treatment of HIV, which have been crucial in viral suppression and increased patient survival. Furthermore, antiviral molecules, such as polymerase inhibitors, have been developed to combat hepatitis C infections by inhibiting viral replication.
Another notable example is the development of broad-acting antivirals, which have shown efficacy against a variety of viruses, including Ebola, Coronavirus, and Respiratory Syncytial Virus. These molecules act by inhibiting viral replication and have been studied as potential treatments for emerging disease outbreaks.
Challenges and prospects:
While progress in discovering antiviral molecules is promising, there are still significant challenges to overcome. Viral resistance is a major concern, as viruses can develop mutations that make antiviral molecules less effective. In addition, the toxicity and side effects of antiviral drugs are issues to be considered during development.
However, advances in biotechnology and gene therapy have the potential to drive the discovery of new antiviral molecules and innovative therapies. RNA-based therapy, for example, is emerging as a promising approach to treating viral infections, using RNA molecules to silence specific viral genes.
Research and development of antiviral molecules have played a crucial role in combating viral infections. Recent scientific advances have allowed the identification of more effective therapeutic targets and the development of antiviral drugs that show promise in treating a variety of viral illnesses.
While challenges remain, the prospects are encouraging, offering hope for better prevention and treatment of viral infections in the future.
Crop Approach
Crop Biolabs is an integrated external R&D facility, specializing in a variety of preclinical and analytical services, external quality assessment, phenotypic and molecular assays for pharmaceuticals, advanced therapeutics, health products, medical devices, cosmetics ingredients, and sanitizing companies, in addition to providing claim substantiation and safety evaluations from discovery to regulation for new solutions and bespoke products.
Our product development team is committed to evaluating the quality and safety of medicines and offers a variety of in vitro preclinical tests per national and international regulatory standards.
Get in touch with us and find out how we can contribute: https://cropbiolabs.com.br/contact-us/
Author: Carolina Barizan, Biomedical Scientist – Marketing Analyst, Crop Biolabs
Crop Biolabs, Bringing Science to People