Advanced therapy products are innovative pharmaceutical products, usually employing live cells, tissues, or genes, that aim to provide more effective and personalized treatments, offering solutions for serious or chronic medical conditions that are not adequately treated by conventional therapies.
Advanced therapy products are classified into three main categories: gene therapy, cell therapy, and tissue engineering products. These therapeutic approaches are in constant development and progress and therefore require accurate safety and efficacy validations before their approval and clinical use.
Assessing the quality of advanced therapy products is extremely important to ensure the effectiveness, safety, and compliance of these products. This quality control and validation can go through different methodologies, which must follow specific guidelines and regulations of the corresponding regulatory bodies and agencies, such as ANVISA (National Health Surveillance Agency), FDA (Food and Drug Administration), and EMA (European Medicines Agency), which provide detailed guidance on quality requirements for advanced therapy products.
In this article, we will address the main strategies and methodologies for validation and quality control of advanced therapy products, in line with regulatory norms and state-of-the-art technologies produced and used nationally and internationally.
Quality control and validation methodologies:
There are several methodologies and in vitro and molecular techniques for validation and quality control of advanced therapy products. Below we will discuss the main ones: Cell differentiation assay, quantification of impurities and metabolites, detection of mycoplasma, sterility test, analysis of endotoxins, detection, and quantification of DMSO, penicillin and streptomycin, analysis of total proteins, immunophenotyping of MSCs, and molecular virology.
Cell differentiation assay
The cell differentiation assay is an important methodology for the quality control of advanced therapy products, especially those involving cell therapy or tissue engineering products. This test makes it possible to assess the ability of cells to differentiate into specific cell types, according to the desired therapeutic purpose.
Cell differentiation refers to the process by which undifferentiated cells develop into specialized cells with specific functions. In the context of advanced therapy products, it is essential to ensure that the cells used are capable of correctly differentiating into cell types relevant to the therapy in question. For example, if mesenchymal stem cells are to be used to treat tissue damage, they must differentiate into target tissue cells such as osteocytes, chondrocytes, or adipocytes.
The cell differentiation assay usually involves the following steps:
- Culture of undifferentiated cells:
The cells are cultivated under specific conditions that favor the maintenance of their undifferentiated state, usually in specific culture media and appropriate culture plates.
- Induction of differentiation:
Cells are exposed to specific stimuli or growth factors that promote differentiation into desired cell types. These stimuli may include chemical compounds, co-culture with other cells, and modification of the culture environment, among others.
- Differentiation assessment:
After a period of exposure to differentiation stimuli, cells are analyzed to see if they have acquired morphological characteristics, surface markers, or gene expression associated with the desired cell type. This can be accomplished using techniques such as immunofluorescence, flow cytometry, polymerase chain reaction (PCR), and gene expression analysis.
- Quantification of differentiation:
In some cases, it is necessary to quantify the degree of cell differentiation. This can be done by counting differentiated cells concerning the total number of cells or employing specific assays for differentiation markers.
Through the Evaluation and photo-documentation of cellular differentiation by inverted microscopy, it is possible to identify it in three cell lines: osteogenesis (staining with Alizarin Red S), adipogenesis (Oil Red staining), and chondrogenesis (toluidine blue staining), as illustrated in the figure below. The image was taken by one of the Crop Biolabs studies:
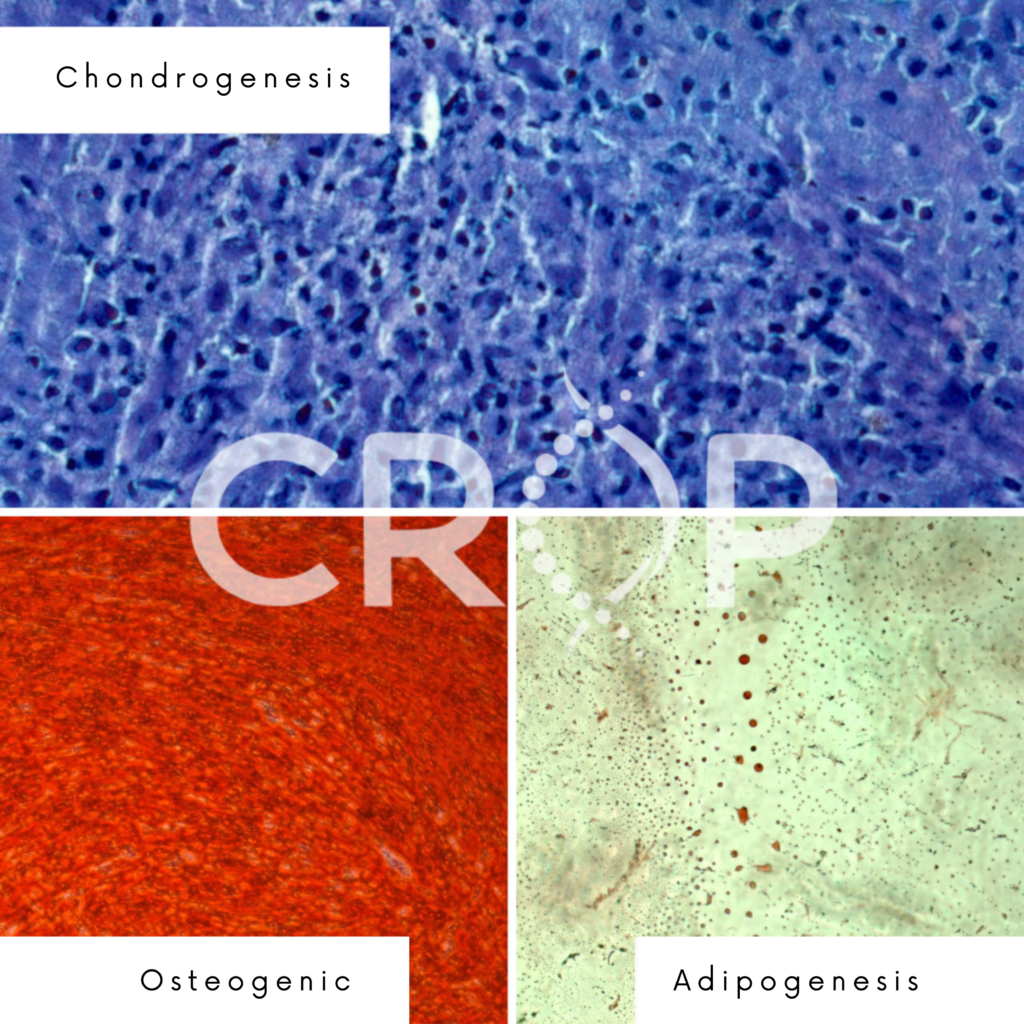
This test makes it possible to assess whether the cells used in the advanced therapy product can differentiate properly and thus perform the desired therapeutic function. It is essential to establish quality criteria and pre-defined specifications for cell differentiation, ensuring the consistency and effectiveness of the final product.
Quantification of Impurities and Metabolites
Another valuable methodology for validation and quality control of advanced therapy products is the quantification of impurities and metabolites, which aims to identify and quantify the presence of undesirable impurities, as well as to evaluate the presence of metabolites that may affect safety and efficacy. of product.
This methodology can be performed using several analytical techniques, depending on the nature of the impurities or metabolites to be quantified, such as enzymatic assays or immunoassays, liquid chromatography, and mass spectrometry.
The liquid chromatography technique coupled with mass spectrometry (LC-MS) has been widely used in the evaluation of impurities and metabolites quantification, as it offers high sensitivity, selectivity, and ability to identify compounds present in complex samples.
Liquid Chromatography (LC) is employed to separate sample components based on their chemical characteristics such as polarity, size, and stationary phase affinity. There are different LC modes that can be used, such as reversed-phase liquid chromatography (RP-LC), ion exchange chromatography (IC), and molecular exclusion chromatography (SEC), among others. The choice of LC mode depends on the characteristics of the compounds to be analyzed.
After chromatographic separation, the eluted compounds are directed to Mass Spectrometry (MS), in which the ionization of the compounds occurs and their fragmentation into ions, which are subsequently detected. MS allows the identification of compounds based on their molecular masses and mass-to-charge ratios (m/z). Furthermore, the LC-MS technique makes it possible to quantify the compounds present in the sample through the relationship between the signal intensity and the known concentration of an internal or external standard.
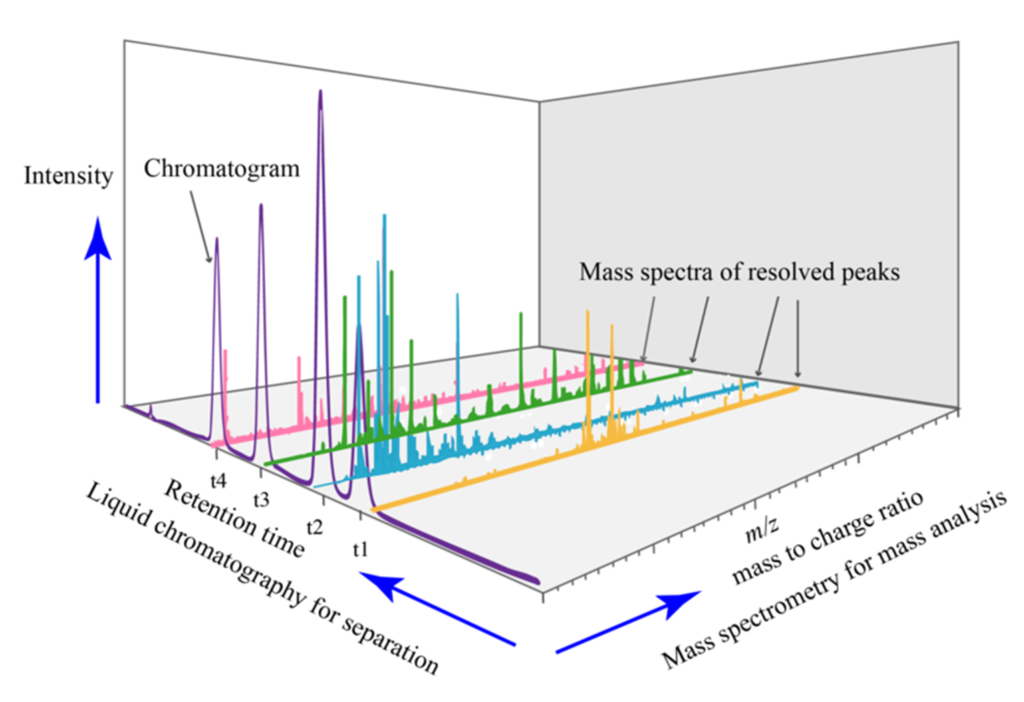
In the context of quality control of advanced therapies, LC-MS can be used to quantify impurities, such as reagent residues or degradation products, that may be present in products. Furthermore, the technique can be applied to quantify endogenous or exogenous metabolites produced by therapeutic cells or tissues after administration of the product.
Importantly, LC-MS requires proper sample preparation, including extraction, purification, and pre-concentration of the compounds of interest. Furthermore, it is necessary to use reference standards for accurate quantification of analytes. Validation of the LC-MS methodology is essential to ensure the robustness, sensitivity, and accuracy of the results obtained.
The precise use of the LC-MS technique provides the detection and quantification of lactate, glutamine/glutamate, ammonia, and glucose in cell culture supernatant samples, which are important indicators of cell metabolism (reflecting the activity and health of therapeutic cells) and may be associated with impurities and unwanted degradation products in advanced therapy products. Thus, the detection of these indicators is important in the evaluation of cell metabolism and the quality of the culture medium, contributing to the quality control and safety of advanced therapies.
More details about the detection and quantification of impurities and metabolites can be found in the EMA’s International Council for Harmonization of Technical Requirements Registration Pharmaceuticals Human Use (ICH) Topic Q3.
Mycoplasma detection
Mycoplasma detection is also an important methodology for the quality control of advanced therapy products. This is because mycoplasmas are microscopic organisms that can contaminate cell cultures and represent a significant concern in compromising the safety and efficacy of products.
Mycoplasma contamination can occur during cell cultivation or through external sources such as reagents, culture medium, or cross-contamination. Mycoplasmas can cause changes in cell physiology, inhibiting growth, impairing viability, and interfering with the functional characteristics of therapeutic cells.
It is important to highlight that mycoplasma detection is a regulatory requirement for advanced therapy products, being required by regulatory agencies. In addition, it is essential to carry out regular tests for the detection of mycoplasma during the development, production, and quality control of advanced therapy products, guaranteeing their safety and quality.
There are several techniques available for the detection of mycoplasma in advanced therapy products, such as culture in selective medium, DAPI staining (4′,6-diamidino-2-phenylindole), Nucleic Acid Hybridization Assay, and RT-qPCR.
The molecular technique of RT-qPCR (Real-time polymerase chain reaction) is widely used for the detection of mycoplasma, detecting the presence of gDNA with specific primers for 16S-23S intergenic regions of Mycoplasma species with the highest incidence of cell culture contamination (M. arginine, M. fermentans, M. hominis, M. hyorhinis, M. orale, M. salivarium, M. pirum, A. laidlawii).
In the “FDA Points to Consider in the Characterization of Cell Lines Used to Produce Biologicals.” it is possible to appreciate more details about Mycoplasma detection.
Sterility test
Sterility testing is another key methodology in the quality control of advanced therapy products. It aims to verify that a product is free of viable microorganisms, such as bacteria, fungi, and viruses, which can cause infections or compromise patient safety.
Advanced therapy products such as cell therapy, gene therapy, and tissue engineering products are produced in complex environments and may be exposed to contamination risks during various stages of the process, from the collection of raw materials to manipulation in the laboratory, making the sterility test essential in guaranteeing the quality and safety of these products.
There are different methods to perform the sterility test, the most common being:
- Membrane Filter Method:
In this method, a sample of the product is filtered through a membrane with pores of a defined size, usually 0.45 or 0.2 micrometers. The membrane retains the microorganisms present in the sample, which is subsequently incubated in appropriate culture media to allow the microorganisms to grow. The absence of growth after the incubation period indicates that the product is sterile.
- Direct Inoculation Method:
In this method, a specific amount of the product is transferred to a sterile and appropriate culture medium. The culture medium is incubated and observed for the growth of microorganisms over time. The absence of growth after incubation indicates the sterility of the product.
The detection of anaerobic and aerobic bacteria, yeasts, and fungi is often performed by inoculum of samples in thioglycollate broth and soy casein in the sterility test for quality control of advanced therapies. This is because these culture media favor the growth of different types of microorganisms and increase the sensitivity of the sterility test.
Thioglycolate Broth is an enriched culture medium that provides suitable conditions for the growth of a wide variety of microorganisms, including anaerobic and aerobic bacteria. This medium contains nutrients that support the growth of both microorganisms that require oxygen and those that grow best in the absence of oxygen. Therefore, the inoculum of samples in thioglycollate broth allows the detection of both aerobic and anaerobic bacteria present in advanced therapy products.
Soybean casein is another culture medium used for sterility testing. This medium is rich in nutrients and allows the growth of bacteria, yeasts, and fungi. The inoculum of samples in soy casein makes it possible to detect contamination by these microorganisms.
In the sterility test, advanced therapy product samples are inoculated into vials containing thioglycolate broth and soy casein. Flasks are incubated under suitable conditions, such as controlled temperature and atmosphere, for a specified time. After incubation, the vials are observed for microbial growth such as turbidity or formation of visible colonies.
Detection of microbial growth in the culture media indicates that the product is not sterile, indicating the presence of contamination. While the absence of microbial growth, and its sterility.
It is important to note that regulatory requirements and guidelines for these procedures may vary between different advanced therapy products. Furthermore, validation of these methods is essential to ensure their effectiveness and reliability.
The detection of anaerobic and aerobic bacteria, yeasts, and fungi through the inoculum of samples in thioglycollate broth and soy casein in the sterility test is a widely used and recognized approach for the quality control of advanced therapies and allows for identifying microbial contamination that can compromise the safety and efficacy of the products.
Regularly performing sterility testing on advanced therapy products ensures that the products are free of viable microorganisms, reducing the risk of infection and ensuring patient safety. It is a crucial step in quality control to ensure that products meet the standards required by regulatory authorities and good manufacturing practices.
Endotoxin analysis
Endotoxin analysis is another vital methodology for the quality control of advanced therapy products. Because they are lipopolysaccharide (LPS) bacterial components present in the cell walls of Gram-negative bacteria, the presence of endotoxins in therapeutic products can pose a significant risk to patient safety, as they have the potential to trigger adverse inflammatory and immunological reactions.
Endotoxin analysis is usually performed using the Gel Clot Limulus amebocyte lysate (LAL) method, which is based on the ability of endotoxins to activate the Limulus amebocyte lysate (LAL) coagulation cascade. LAL is a reagent derived from the blood of the horseshoe crab (Limulus polyphemus), which contains amebocytes (blood cells) sensitive to endotoxins. When endotoxins are present, clotting of the LAL occurs, which can be quantified and used to determine the amount of endotoxins present in the sample.
Endotoxin analysis is especially relevant for advanced therapy products that involve biological materials such as cell cultures, culture media, storage solutions, and medical devices. These materials can be potential sources of bacterial contamination and therefore may contain endotoxins.
Allowable endotoxin limits in advanced therapy products are set by regulatory agencies and therefore must be tested for the presence of endotoxins according to specific regulatory requirements.
It is important to mention that the analysis of endotoxins must be performed under-recognized methods and standards, such as chapter <85> of the United States Pharmacopoeia (USP) and chapter 2.6.14 of the European Pharmacopoeia (EP). In addition, it is necessary to ensure method validation, including verification of test sensitivity and specificity.
Detection and quantification of dimethyl sulfoxide (DMSO)
The detection and quantification of dimethyl sulfoxide (DMSO) can form part of the quality control of therapy products, especially for those that use DMSO as an important component in the manufacturing or preservation process.
DMSO is a widely used solvent in biological research and advanced therapies. It can be used as a cryoprotectant, helping to preserve cells and tissues during the freezing process and long-term storage. However, it is important to ensure that final products do not overdo it with excessive levels of DMSO, as high concentrations can have toxic effects on cells and tissues.
The detection and quantification of DMSO can be performed through the technique of high-performance liquid chromatography (HPLC) coupled with mass spectrometry (MS), allowing the separation and identification of DMSO in the sample, as well as the precise quantification of its concentration.
In the quality control process, product samples are prepared and analyzed following applicable regulatory requirements and guidelines, establishing acceptable limits for DMSO concentration based on safety and efficacy studies. Samples of the tested product are compared to these safety limits to ensure compliance.
By keeping DMSO concentrations within established limits, it is possible to minimize its toxic effects and preserve the viability and functionality of cells and tissues used in advanced therapies, making the DMSO detection and quantification assay an essential part of product quality control who uses it.
Importantly, the exact methodology for detecting and quantifying DMSO may vary depending on specific product requirements and applicable regulatory guidelines. Therefore, it is critical to follow proper practices and validated methods to ensure reliable and accurate results.
Detection of penicillin and streptomycin
Like the DMSO detection and quantification assay, the penicillin and streptomycin detection test becomes an essential part of the quality control of advanced therapy products that use them during their manufacturing process or if there is the possibility of cross-contamination with these substances.
Penicillin and streptomycin are antibiotics widely used in the treatment of bacterial infections. However, in certain cases, the presence of these antibiotics in therapeutic products may be undesirable.
For the detection of penicillin and streptomycin, the most widely used technique is also liquid chromatography coupled to mass spectrometry (LC-MS), which enables the process of separation and identification of antibiotics in the sample, as well as the precise quantification of their concentrations.
Total protein analysis (BCA)
The analysis of total proteins using the BCA (copper bicinchoninate) method can be an important methodology for the quality control of advanced therapy products. The quantification of total proteins is relevant, as it provides information on the concentration of proteins present in the product, which is important to ensure the consistency and quality of the final product.
The BCA method is a colorimetric technique based on the reaction between the protein present in the sample and the BCA reagent. The reagent forms a colored complex with proteins in solution, and the color intensity is directly proportional to the concentration of proteins present. This intensity can be measured using techniques such as Western-Blot to quantify the concentration of proteins in the sample.
Total protein analysis using the BCA method can be performed at various stages of the advanced therapy product manufacturing process. For example, it is common to perform this analysis to verify the concentration of proteins in culture media, storage solutions, and products derived from cells or tissues, among others.
When performing total protein analysis, it is important to follow proper procedures and establish a validated protocol. This involves correct sample preparation, the addition of the BCA reagent, and accurate spectrophotometric reading to quantify protein concentration. Furthermore, it is important to use a reference protein standard to calibrate the calibration curve and ensure reliable and comparable results.
Immunophenotyping Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are multipotent cells capable of differentiating into several cell types, being widely used in research and therapeutic applications due to their regenerative and immunomodulatory properties.
MSC immunophenotyping is a process that involves marking and detecting of specific antigens on the surface of MSCs using monoclonal antibodies. These antibodies can be conjugated to fluorescent or enzymatic markers to allow the identification and characterization of MSCs. Through immunophenotyping, it is possible to assess the presence or absence of specific surface markers in MSCs, which helps in the identification and characterization of these cells.
In the quality control of advanced therapies using MSCs, immunophenotyping is widely used to confirm the identity of cells and assess their purity and viability. This is especially important because the presence of other contaminating cells can affect the safety and efficacy of the final product.
Through immunophenotyping, it is possible to identify specific markers of MSCs, such as CD73, CD90, and CD105, and exclude the presence of undesirable cell markers, such as CD45 (a marker of immune system cells) and CD34 (a marker of hematopoietic progenitor cells).
In addition, immunophenotyping can also be used to monitor the quality and consistency of MSCs over time, allowing the assessment of changes in the phenotypic characteristics of cells during in vitro expansion. This is important to ensure the stability of CTMs and maintain compliance with quality and effectiveness requirements.
Immunophenotyping of MSCs is performed using flow cytometry, a technique that allows simultaneous analysis of multiple fluorescence parameters in individual cells. Immunophenotyping results are interpreted by comparing marker expression patterns in MSCs with appropriate controls and with known phenotypic profiles of MSCs, with the interpretation of positivity when ≥ 95% positive for CD73, CD90, and CD105 and ≤ 2% negative for CD34, CD45, CD11b or CD14, CD19 or CD79α and HLA-DR (International Society for Cell Therapy).
Molecular virology
Molecular virology plays an essential role in the quality control of advanced therapies, allowing the detection and quantification of nucleic acids from different viruses present in therapeutic products. For this, several techniques are used, such as RT-qPCR, TCID50 (infective dose 50%), and infectious viral titer.
RT-qPCR is a sensitive and specific technique that allows the detection and quantification of viral nucleic acids in samples. It combines reverse transcription (RT), which converts viral RNA into complementary DNA (cDNA), and real-time PCR, which amplifies and quantifies viral cDNA. RT-qPCR is commonly used for the detection of viruses such as CMV (cytomegalovirus), HIV-1 and HIV-2, HTLV-I and HTLV-II, EBV (Epstein-Barr virus), HBV (hepatitis B virus), HCV (hepatitis C virus) and B19 (human parvovirus B19).
The TCID50 technique is used to determine the concentration of infectious viral particles in a sample. TCID50 stands for “50% infecting dose”, which is the sample dilution that results in 50% infected cells. This method is often used to assess the infectivity of viruses in therapeutic products such as cell cultures or viral preparations.
The infectious viral titer is another approach to quantifying the concentration of infectious viral particles. This method measures the amount of virus capable of infecting target cells in a sample. It usually involves serial dilution of the sample, inoculation into appropriate cells, and evaluation of the cytopathic effect (cell death or change) after a given incubation period. The infectious viral titer is particularly useful for assessing the presence and activity of infectious viruses in advanced therapies.
The use of these molecular virology techniques, such as RT-qPCR, TCID50, and infectious viral titers, in the quality control of advanced therapies allows the detection, quantification, and evaluation of viral activity in therapeutic products. These methodologies are essential to ensure product safety and minimize the risk of viral transmission to patients.
Crop Approach
Crop Biolabs is an integrated external R&D facility, specializing in a variety of preclinical and analytical services, external quality assessment, and phenotypic and molecular assays for cell-based therapeutics, pharmaceuticals, cosmetic ingredients, and sanitizing companies, in addition to providing claim substantiation and safety evaluations from discovery to regulation for new solutions and bespoke products.
Our product development team offers Quality Control and validation for cell-based therapies aligned with the regulatory standards of ANVISA, FDA, and EMA, and includes all the methodologies presented in the article in its portfolio (cell differentiation assay, quantification of impurities and metabolites, detection of mycoplasma, sterility test, analysis of endotoxins, detection, and quantification of DMSO, penicillin and streptomycin, analysis of total proteins, immunophenotyping of MSCs and molecular virology), with a personalized, agile, cost-effective and human-way.
Our mission is to collaborate with the technological maturation of products and developments in life sciences, either with a punctual service or with a complete R&D solution: from idea to impact.
Learn more about Crop Biolabs and get in touch. Let’s work together: https://cropbiolabs.com.br/
References:
- FDA Points to Consider in the Characterization of Cell Lines Used to Produce Biologicals;
- Brazilian Pharmacopoeia, 6th edition sterility test (5.5.3.2.1);
- FDA Center for Biologics Evaluation and Research ADVANCING REGULATORY SCIENCE – Office of Tissues and Advanced Therapies Division of Cellular and Gene Therapies Cellular and Tissue Therapy Branch;
- EMA Stem cell-based medicinal products – Scientific guideline.
Author: Carolina Barizan, Biomedical Scientist – Marketing Analyst, Crop Biolabs