Global trend
Alternative methodologies have been increasingly expected in safety and efficacy estimates around the world. Since 2009, animal testing for cosmetics has been banned in laboratories in European countries and since 2013 the marketing of cosmetic products tested on animals has been prohibited.
In Brazil, 8 states already prohibit testing on animals for cosmetics and a legislative signature is in the process so that the retention extends over the entire national territory. Currently, ANVISA (National Health Surveillance Agency) considers 18 alternative methods as validated.
In the United States of America (USA), the regulatory bodies FDA (Food and Drug Administration) and NIH (National Institutes of Health) have the objective of completely abolishing the use of animals by 2035, both for cosmetics and pharmaceuticals. Currently, not all regulatory tests are available for alternative methods, but the goal is to create them by 2035, meeting the demand.
China still uses many animals for regulatory testing, but external pressure from the global cruelty-free trend has also impacted the country’s stance on animal use over the last decade. In 2014 declared that animal testing for cosmetic products is no longer necessary.
Faced with these and other different laws regarding the use of animals for experimentation around the world, the ICCR (International Cooperation on Cosmetics Regulation) was developed in 2007 as an international group of regulatory authorities from Brazil, Canada, the European Union, Japan, China, Korea, United States of America and Israel, to discuss the common challenges of regulatory safety testing for cosmetics and to harmonize the different legislations around the world regarding the use of alternative methods.
Every year, Great Britain provides a statistical report on the use of animals in scientific procedures. In the 2021 annual report, it is possible to observe the most frequently used species of animals and which purposes of scientific research most use animals in tests, as shown in the graphs below:
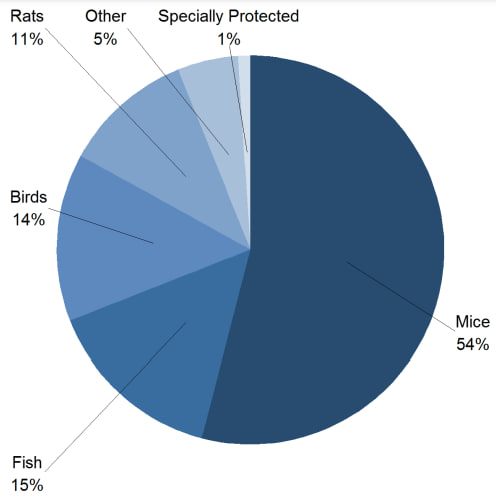
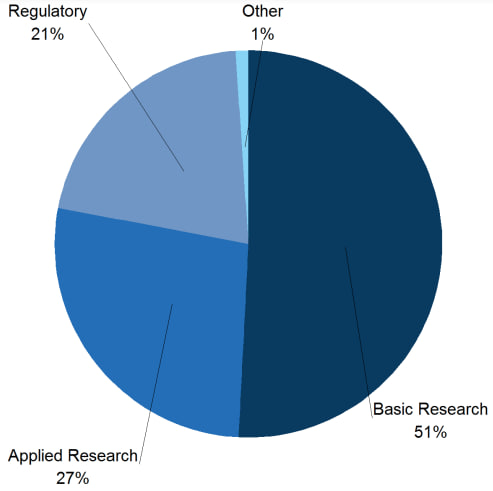
As noted, mice continue to be the animal most used in experimentation, and basic research, largely produced by universities, is still the one that most uses animals, also presenting a greater challenge for replacing the models.
Now, because of the advances that alternative methodologies have shown, a reflection has accelerated the transformations of companies, industries, and scientific producers in general: we can no longer use outdated technologies, especially when it comes to health.
With that in mind and in presenting a paradigm shift for toxicological tests, John Hopkins University developed the course “Toxicology of the 21st Century”, instigating alternative methodologies for safety tests such as the “Organ-on-a-chip”. But before going into more detail about alternative methodologies, let’s reinforce some toxicology concepts.
Toxicological studies
“The dose makes the poison”
16th-century physician and father of toxicology Paracelso
According to Paracelso, every substance can become a poison depending on its dosage, corroborated by the concept of LD50 (Lethal Dose 50), established in 1920 to define the acute toxicity threshold of a substance capable of killing 50% of tested animals.
In this sense, efficacy and toxicology can be considered brothers, depending on the dosage, mode of use, and exposure.
To ensure the safety of a chemical substance, therefore, it is necessary to carry out toxicological tests with the following objectives:
- Identify adverse effects;
- Develop dose-response relationship;
- Predict the effects of human exposure.
Currently, it is already possible to identify adverse effects and develop the dose-response relationship using the alternative methods available, but it is still not possible to predict the effects of human exposure through the alternative methodologies developed so far.
Alternative Methodologies
Based on the 3Rs Principle (Reduction, Replacing, and Refinement), the alternative methodologies aim to replace the use of animals in experimentation with validated methods of high specificity and sensitivity that present a correlation with tests in animals and humans.
Being widely used in toxicological and ocular and skin irritation and corrosion tests, for example, alternative methods have also been used a lot for the evaluation of endocrine disruption, a rising demand, for the observation of a possible imbalance in the endocrine organs due to contact with substances, such as essential oils.
But before exploring the available alternative methodologies, it is still necessary to understand the difference between valid and validated methods.
Valid methods are those with proven integrity and relevance, but which have not gone through the regulatory approval process.
Validated methods are those that have gone through the validation process, have been reviewed by the ESAC (European Surveillance of Antimicrobial Consumption); approved by the Scientific Committee on Consumer Safety (SCCS); and approved and described in the OECD (The Organization for Economic Co-operation and Development).
They are the validated alternative methods that can be used as a substitute for the use of animals in the experimental design so that their safety evaluation results can be accepted.
It is important to point out that if the methodology is validated for chemical substances, then it covers varied markets: cosmetics; pharmaceuticals; agrochemicals, among others. Thus, all products that are a mixture of chemical substances, such as cosmetics, must also comply with REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals).
Having understood what the validated alternative methodologies are, let’s get to know some of them that are key in toxicological evaluations, such as Adverse outcome pathway (AOP); New Approach Methodologies (NAMs); Next Generation of Risk Assessments (NGRA); QSAR models and read-across; and Weight of Evidence (WOE).
Adverse Outcome Pathway (AOP):
AOPs are a core element of toxicology, built to support risk assessment of a chemical substance based on mechanistic reasoning. It is an analytical construct that describes a sequential chain of events at different levels of biological organization, resulting in an adverse effect, as indicated below:
Using the AOP as a validation process, it is possible to assess adverse effects such as skin irritation by considering, for example, which path, the steps, from skin contact with a substance to the development of an itch in the contact region. It is considering the AOPs that the integrated tests are developed. You can find more details on the use of AOP in OECD 260 (2016).
AOP Kiwi reports on projects in several countries, organized by the OECD, for the identification and characterization of AOPs.
New Approach Methodologies (NAMs):
NAMs are technologies and approaches, including computational modeling; in vitro assays; and testing using alternative animal species, can inform hazard and risk assessment decisions without animal testing.
Next Generation of Risk Assessments (NGRA):
NGRA is an exposure-led, hypothesis-driven risk assessment approach that integrates in silico, in chemico, and in vitro approaches.
It is important to point out that to work with NAMs and NGRA it is necessary to know the AOP first.
QSAR models and read-across:
OECD QSAR ToolBox free-access is a software designed to support hazard assessment of chemicals as well as to increase mechanistic and other knowledge on chemical substances in a cost-efficient way. There is still a lack of data in this bank; sometimes it is necessary to complement them in the study with other databases.
Weight of evidence (WOE):
WOE is the interpretation of toxicological data in the context of all available information assessing the strength and quality of evidence relating to a decision.
Another trend presented as the future of alternative methods is the Organ-on-chip, in which evaluations are carried out no longer only through tissue reconstitution but using microfluidics. A technique that makes it possible to evaluate different cell types on a single chip.
In short, alternative methodologies have increasingly presented themselves as a global demand for experimental development, offering various techniques and specific and sensitive models and corroborating the decrease in the use of animals in research and development, in addition to supplying potentially outdated technologies in evaluations of safety and efficacy of chemical substances, representing the current moment of cutting-edge technology developed in laboratories around the world, and a potential glimpse of new technologies to be developed.
All Crop Biolabs services are performed with validated alternative methods, guaranteeing totally cruelty-free experimental designs. Contact our team and learn more: https://cropbiolabs.com.br/contact-us/
References:
Annual statistics of scientific procedures on living animals, Great Britain 2021: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1118195/annual-statistics-scientific-procedures-living-animals-2021_161122_v5.pdf
GUIDANCE DOCUMENT FOR THE USE OF ADVERSE OUTCOME PATHWAYS IN DEVELOPING INTEGRATED APPROACHES TO TESTING AND ASSESSMENT (IATA), OECD No 260, 2016
The OECD QSAR Toolbox: https://www.oecd.org/chemicalsafety/risk-assessment/oecd-qsar-toolbox.htm
AOP Kiwi: https://aopwiki.org/
Author: Carolina Barizan Perdão, Biomedical Scientist – Marketing Analyst, Crop Biolabs