“Weight of evidence” (WOE) is a relevant toxicological tool, as well as Adverse outcome pathway (AOP); New Approach Methodoloies (NAMs) and Next Generation Risk Assessment (NGRA), thus being a common term in the scientific literature, most often seen in the context of risk assessments (RA). However, the definition of this concept still causes doubts.
During 10 years (1994-2004), the term “Weight of evidence” was defined by publications on PubMed in three main characteristics:
- Metaphorical: WOE refers to a collection of studies or an unspecified methodological approach;
- Methodological: WOE pointing to established interpretative methodologies (e.g., systematic narrative review, meta-analysis, causal criteria, or quality criteria for toxicological studies) or meaning that “all” rather than some subset of the evidence is examined, or rarely, pointing to methods using quantitative weights for evidence.
- Theoretical: WOE serves as a label for a conceptual framework.
In the last WOE definition, as Theoretical, some problems are identified:
- The frequent lack of definition of the term “weight of evidence;
- Multiple uses of the term and a lack of consensus about its meaning;
- Many different kinds of weights, both qualitative and quantitative, can be used in risk assessments (RA).
Thus, a recommendation of practice for publishing is to describe the WOE concept and its associated methods in the experimental design.
New Methodologies
A new strategy in Risk Assessments has been used for the definition and decision-making of the Weight of Evidence (WOE), which is a combination of New Approach Methodologies (NAMs) and new technology with historical animal data.
In silico methods have also been used as part of the weight of proof (WOE) through some of the currently available models and high-quality tools, providing additional supporting evidence. However, there are still limitations regarding the official validation of these methods.
According to the Scientific Committee on Consumer Safety (SCCS), the results of in silico toxicity assessment are more useful for hazard assessment when they are integrated with other sources of evidence (e.g. in vitro results) into an overall weight of evidence (WoE).
Another indicator test that could contribute to the weight of evidence (WOE) approach is the Genotoxicity test, which measures DNA damage without taking into account the consequences of this primary damage. However, it should not be used as a stand-alone test for the WOE.
In silico methods and models point to the potential to generate supporting evidence on the mutagenicity and genotoxicity of substances, supporting WoE in its safety together with other in vitro data.
As indicated in section 3.4.2 of the 11th edition SCCS, estimates derived from in silico and comparative models can provide useful information to further support evidence in the hazard assessment, especially when the results are integrated with other sources of evidence to then form the overall weight of evidence (WoE).
See in the figure below three basic steps to reach the overall weight of evidence, integrating the sources of evidence:
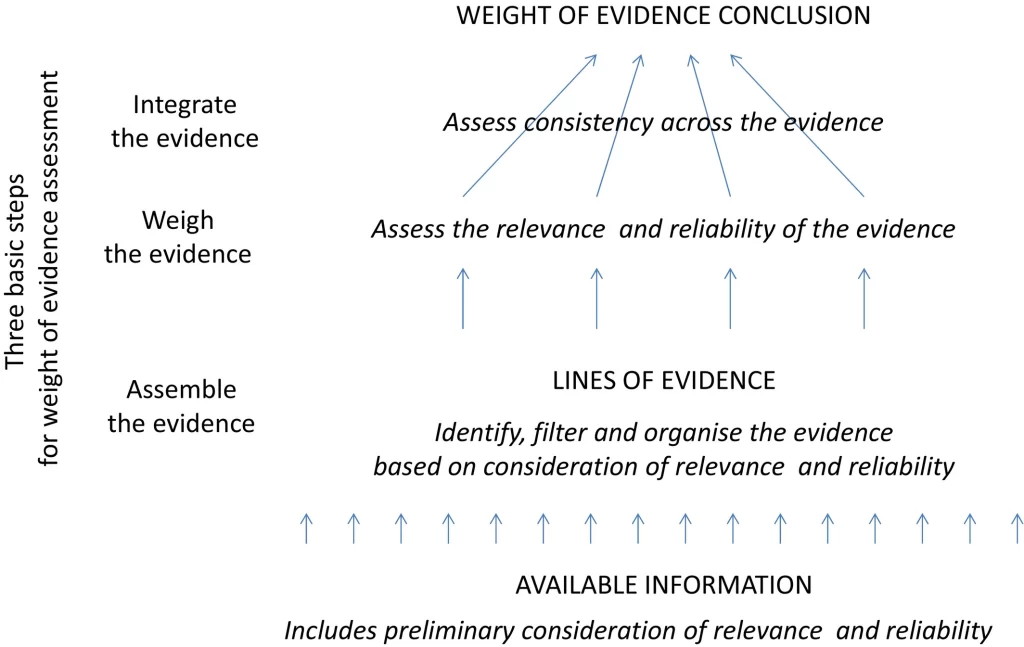
Before carrying out any test, a complete review of all available data on the substance under evaluation must be carried out, defining all parameters and variables that will be used as the weight of evidence (WOE). The next figure presents the diference betwwing a classic systematic review and a classic weight of evidence pathway.
References:
Guidance on the use of the weight of evidence approach in scientific assessments, EFSA Scientific Committee. https://doi.org/10.2903/j.efsa.2017.4971
Glenn Suter, Office of Research and Development, Emeritus, U.S. Environmental Protection Agency, Cincinnati, Ohio, USA, 2020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7551547/
THE SCCS NOTES OF GUIDANCE FOR THE TESTING OF COSMETIC INGREDIENTS AND THEIR SAFETY EVALUATION 11TH REVISION
Author: Carolina Barizan Perdão, Biomedical Scientist. Marketing Analyst, Crop Biolabs