There is a commitment from the world scientific community to follow the Principles of Russell-Burch (1959) of “reduction, replacement, and refinement” in the use of animals, known as the Principle of the 3Rs or Principles of Humane Experimental Technique.
In the present update, the state-of-the-art concerning the validated methods of the 3Rs (Refinement, Reduction, and Replacement) strategy of Russell et al. (1959) is incorporated with emphasis on New Approach Methodologies (NAMs) – technologies and approaches (including computational modeling, in vitro assays, and testing using alternative animal species) that can inform hazard and risk assessment decisions without the use of animal testing.
In view of the testing and marketing bans in cosmetic regulation, for example, the Scientific Committee on Consumer Safety Guidance (SCCS) gives special attention to those alternative methods that are suitable for the safety testing of cosmetic substances. New methodologies for risk assessment of chemicals without using animal experimentation are being explored worldwide.
Attention is given here to Next-Generation Risk Assessment (NGRA) as a possible framework for the safety evaluation of cosmetic ingredients and the NAMs that would fit into this structure (Rogiers et al., 2020). Risk assessment of cosmetics and their ingredients is shifting towards a strategic combination of NAMs and new technology with historical animal data, if available, to come to a Weight of Evidence (WoE) decision-making approach.
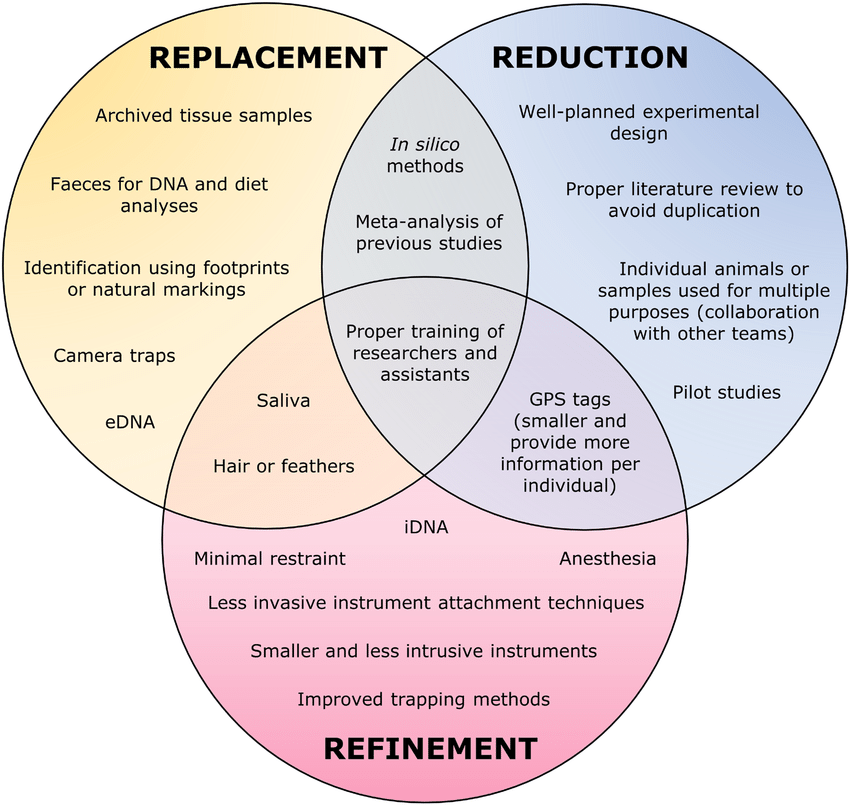
Alternatives for Substitution, Reduction, and Refinement of the use of animals in the experiment:
- Replacement: The term “replacement technique” is used for any scientific method employing non-sentient material which may in the history of experimentation replace methods that use conscious living vertebrates. To substitute, it is possible to opt for higher plants, microorganisms, and the more degenerate metazoan endoparasites, in which nervous and sensory systems are almost atrophied, using in vitro techniques with cell and tissue culture; physical-chemical systems that mimic biological functions and simulate physiological processes using computers.
- Reduction: The replacement progress is gradual, and if it is impossible to absorb all the experimental biology, the reduction in the use of animals may be the way to go. Some actions can contribute to reducing the use of animals even in the experimental design, through the correct choice of strategies in the planning and execution of entire lines of research, such as developing new protocols for experimental screening to investigate interest later and defining the largest number of relevant information to be obtained in the study by the smallest number of animals possible.
- Refinement: Refinement, the third great way of advancement, presents more formidable difficulties. It is indeed so versatile in its aspects that it almost seems to demand a different solution in each investigation, and refinement can be considered an art or a skill to improvise. However, the subject of refinement admits some generalizations. The refinement starts when it is not possible to replace or reduce de use of animals in the experimental design, and its object is simply to reduce to an absolute minimum the amount of distress imposed on those animals that are still used. Some of the generalizations for refining experimental protocols, to minimize pain or animal stress, is essential to pay attention to using appropriate analgesics and anesthetics for potentially painful experiments; Ensuring drug dosages are correct; performing a single surgery per animal, and defining strategies to identify animal pain or stress, establishing procedures to prevent or alleviate them.
At Crop Biolabs, all pre-clinical tests are performed in vitro, cruelty-free, guaranteeing a commitment to the Russell-Burch Principles and innovation in experimental designs, using New Approach Methodologies (NAMs).
Learn more about our services, contact our team, and schedule a meeting: https://cropbiolabs.com.br/contact-us/
References:
- Université de Fribourg, 2020. Zemanova, M.A. DOI: https://doi.org/10.2981/wlb.00607
- THE SCCS NOTES OF GUIDANCE FOR THE TESTING OF COSMETIC INGREDIENTS AND THEIR SAFETY EVALUATION 11TH REVISION
Author: Carolina Barizan Perdão, Marketing Analyst Crop Biolabs