According to the United Nations Globally Harmonized System of Classification and Labeling of Chemicals (UN GHS), skin sensitization refers to an allergic response following skin contact with a chemical.
Following the OECD 442, Guideline for the testing of Chemicals: In Chemico Skin Sensitisation, for the assessment of skin sensitization, it is ideal that there be a prior evaluation of all available information on the composition of the product or active tested, such as data from previous tests in vitro, in vivo, historical human data, valid (Q)SARs ((Quantitative) Structure-Activity Relationships), and structurally related substances.
Thus, in vivo testing is only necessary if in vitro methods are not suitable for the substance or if the in vitro test results are not suitable for classification and risk assessment.
The in vivo test does not need to be conducted If the conditions specified in column 2 of Annex VII Section 8.3 of the REACH Regulation about the substance are met, including:
• Classification of the substance as skin corrosion (Cat 1); or
• Definetion of the substance as a strong acid (pH <2) or base (pH >11.5); or
• Identification of the substance as spontaneously flammable in air, in contact with water, or moisture at room temperature.
Taking this into account, we suggest the following workflow:
- Use the QSAR Toolbox in silico to predict product/asset risks:
The OECD QSAR Toolbox is freely available software co-developed by ECHA (The European Chemicals Agency) and the OECD. It provides tools to carry out or support chemical hazard assessments for most parameters of regulatory interest. Using chemical structure and other identifiers, the software identifies analogs of a target and makes predictions based on automated reading and structural alerts.
- Define the specific key event within the awareness pathway:
The key-specific event in the sensitization pathway may be molecular interaction with skin proteins; inflammatory response in keratinocytes and activation of dendritic cells. Let’s get to know the methods for defining each one:
- Molecular interaction with skin proteins
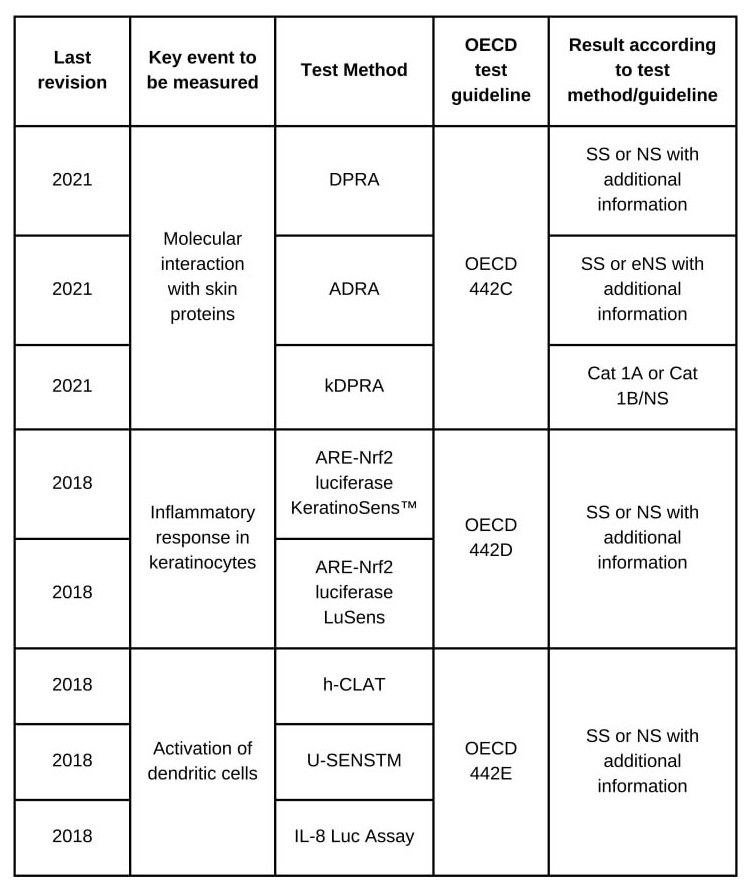
The method for testing molecular interaction with skin proteins corresponds to OECD 442C, containing three chemical assays that address peptide reactivity:
i) Direct Peptide Reactivity Assay (DPRA);
ii) Amino Acid Derivative Reactivity Assay (ADRA);
iii) Kinetic Direct Peptide Reactivity Assay (kDPRA).
The DPRA and ADRA methods identify hazards, and the kDPRA test identifies potent sensitizers (subcategory 1A). The kDPRA assay can be used as a stand-alone test if the result indicates that the product/active is from Subcategory 1A (Strong sensitizing agents Subcategory).
- Inflammatory response in keratinocytes
The method for testing the inflammatory response in keratinocytes corresponds to OECD 442D, containing two tests that use luminescence detection to measure the gene expression of pathways dependent on the antioxidant/electrophile response element (ARE – expression of antioxidant/electrophile response element), being:
i) ARE-Nrf2 luciferase KeratinoSens™ test method;
ii) ARE-Nrf2 luciferase LuSens test method.
- Activation of dendritic cells
The method for testing dendritic cell (DC) activation corresponds to OECD 442E, containing three tests:
i) Human Cell Line Activation Test (h-CLAT), which measures, by flow cytometry, the expression levels of surface markers linked to DC maturation, ie CD86 and CD54;
ii) U937 cell line activation test (U-SENSTM), which measures specific levels of CD86;
iii) Interleukin-8 Reporter Gene Assay (IL-8 Luc Assay), which measures changes in a cytokine linked to DC activation, through detection of IL-8 mRNA.
The table below presents the Sensitization classification result, according to the corresponding method and guideline:
Learn more about In vitro skin sensitization test and other Crop Biolabs services for cosmetics ingredients: https://cropbiolabs.com.br/cosmetic/
References:
- OECD Guidance Document on Reporting of Defined Approaches and individual information sources to be used within Integrated Approaches to Testing and Assessment (IATA) for skin sensitization (OECD GD 256, 2016)
- Guidance on information requirements and chemical safety assessment, section R.7.3 (ECHA Guidance R7a): https://echa.europa.eu/documents/10162/17224/information_requirements_r7a_en.pdf/e4a2a18f-a2bd-4a04-ac6d-0ea425b2567f?t=1500286622893
- Guidance on information requirements and chemical safety assessment (ECHA Guidance R.4): http://echa.europa.eu/documents/10162/13643/information_requirements_r4_en.pdf